Table of Contents
What is Acetate?
Acetate refers to the acetate ion (CH3COO−) or acetate salts, which are compounds containing the acetate ion. The acetate ion is the conjugate base of acetic acid (CH3COOH), a weak acid commonly found in vinegar. Acetate salts are formed by the reaction of acetic acid with a base, resulting in the removal of a proton (H+) from the acid.
Structure Of Acetate (C2H3O2−)
The acetate ion (C2H3O2−), commonly represented as CH3COO−, consists of two parts: the acetyl group (CH3CO−) and the negatively charged oxygen atom (O−). Here’s a simplified representation of the structure of the acetate ion:
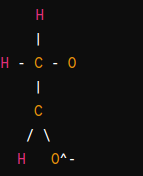
Properties of Acetate
| Acetate | C2H3O2− |
| Monoisotopic Mass of Acetate | 59.013 g/mol |
| Complexity | 25.5 |
| Molecular Weight of Acetate | 59.044 g/mol |
| Conjugate acid | Acetic acid |
Uses of Acetate
- Acetate fibers are used in the production of textiles and clothing, including dresses, blouses, and linings. Acetate fabrics are known for their lustrous appearance and draping qualities.
- Acetate is used in pharmaceutical formulations as a buffering agent and excipient. It helps stabilize pH levels in oral medications, injectable solutions, and topical preparations.
- Acetate salts, such as sodium acetate and potassium acetate, are used as food additives for flavor enhancement, acidity regulation, and preservation. They are commonly found in condiments, pickles, and snack foods.
- Acetate ions serve as versatile building blocks in organic synthesis, participating in various chemical reactions to form new compounds. They are used in the synthesis of pharmaceuticals, plastics, fragrances, and other organic chemicals.
- Acetate-based cleaning solutions are used for removing grease, oil, and dirt from surfaces in household and industrial settings. They are commonly found in degreasers, glass cleaners, and floor strippers.
FAQs
- What is acetate?
- Acetate refers to the acetate ion (CH3COO−) or acetate salts, which are compounds containing this ion. It is derived from acetic acid and is commonly found in various industries and biological systems.
- What is the structure of acetate?
- The acetate ion consists of a carbon atom double-bonded to an oxygen atom (carbonyl group), with one of the carbon’s other bonds being a single bond to another oxygen atom. This oxygen atom carries a negative charge, and there is also a methyl group attached to the same carbon atom.
- What are the properties of acetate?
- Acetate salts are usually white, crystalline solids that are soluble in water. They are weakly basic and can act as buffers in solutions. Acetate ions are also biodegradable and can serve as carbon sources for microorganisms.
- What are the common uses of acetate?
- Acetate finds applications in textiles (e.g., acetate fibers), photography (e.g., cellulose acetate film), pharmaceuticals (e.g., as a buffering agent), food additives (e.g., sodium acetate), chemical synthesis, industrial processes, cleaning products, and laboratory applications.
- Is acetate safe?
- Acetate salts are generally considered safe for consumption in moderate amounts, and they are commonly used in food and pharmaceutical products. However, excessive intake of acetate may lead to health issues, and specific regulations govern their use in various products.
- How is acetate synthesized?
- Acetate salts can be synthesized by the reaction of acetic acid with bases such as sodium hydroxide or by the neutralization of acetic acid with metal hydroxides. Acetate ions can also be produced biologically through metabolic processes.
- What are the environmental implications of acetate?
- Acetate is biodegradable and can serve as a carbon source for microorganisms in natural environments. However, excessive release of acetate into ecosystems can disrupt ecological balance and may contribute to pollution if not properly managed.